|
Project
Organic compounds emitted in the atmosphere are oxidized in complex reactions sequences that produce a myriad of intermediates. Although the cumulative importance of these organic intermediates is widely acknowledged, there is still a critical lack of information concerning the detailed composition of the highly functionalized secondary organics in the gas and condensed phases. The evaluation of thier impacts on the pollution episodes, climate, and the tropospheric oxydizing capacity requires modelling tools that track the identity and reactivity of organic carbon in the various phases down to the ultimate oxidation products, CO and CO2. However, a fully detailed representation of the atmospheric transformations of organic compounds involves a very large number of intermediate species, far in excess of the number that can be reasonably written manually. This website presents a data processing tool to generate the explicit gas-phase oxidation schemes of acyclic hydrocarbons and their oxidation products under tropospheric conditions and the protocol used to select the reaction products and the rate constants.
- VOC oxidation
- The generator protocol
- Using explicit schemes
- VOC oxidation
The construction of the chemical schemes generator is based on identifying
all the reactions for a given primary (i.e. emitted) species as well as for
every intermediate up to CO or CO2 production. Atmospheric
oxidation of organic compounds can be represented by a limited number of
reaction types, repeated many times up to full oxidation of the given parent
compound. With a few exceptions, these steps can be summarized as follow (see Fig.1):
The initiation of the radical organic chains by reaction with OH,
NO3, O3 or via bond breaking after the absorption of a photon.
This first step generally leads to the formation of an organic peroxy radical (RO2). Peroxy radical reactions with NO, NO2, NO3, HO2
or with others RO2 radicals. These reactions either lead to a stable
(i.e.a non radical) secondary organic species or to an organic alkoxy
radical (RO). Alkoxy radical reaction with O2, unimolecular decomposition
(i.e. C-C bond breaking) or isomerization. These reactions either lead to a
stable secondary and/or to a new peroxy radical.
The redundancy in these reaction sequences is the basis of the generator.
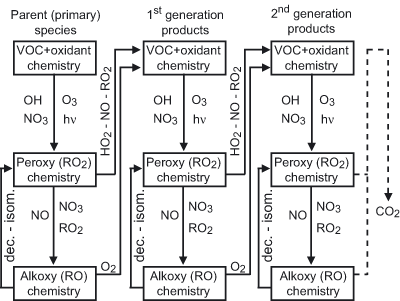 Figure 1 Figure 1
- The generator protocol
The general philosophy followed to develop the generator is similar to the
methods recently described by Saunders. The generator can be
viewed as a computer program that mimics the steps by which chemists might
develop chemical schemes (i.e. writing the list of the reactions involved in
the oxidation of a given species and their associated rate constants). These
steps are illustrated Fig.2 and can be summarized as follow.
The molecular structure of the species is analized and possible reactive pathways are identified. If no experimental data are available, an estimation of the rate constant and reaction products is performed
using structure-activity relationships (SAR). If new, the products are added to a stack for further reactions.
These steps are repeated as long as species are
present in the stack. The process stops when the stack is empty, which occurs
when the full oxidation scheme has been written.
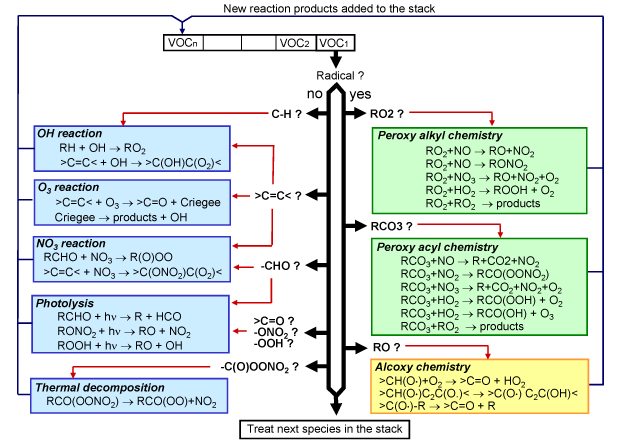 Figure 2 Figure 2
- Using explicit schemes
Explicit schemes could be use as an "exploratory vehicle" to explore the behavior of organic
matter during oxidation. Thanks to those schemes, it is possible to simulate the oxidation of
species and study where the carbon goes. The following picture represents the oxidation of the octane,
simulated during 12 days.
The simulation conditions are: T = 298K, [octane]0 = 20 ppb, [NOx]0 = 10 ppb.
The next graphics are presented in order to study how the organic reactivity evolves.
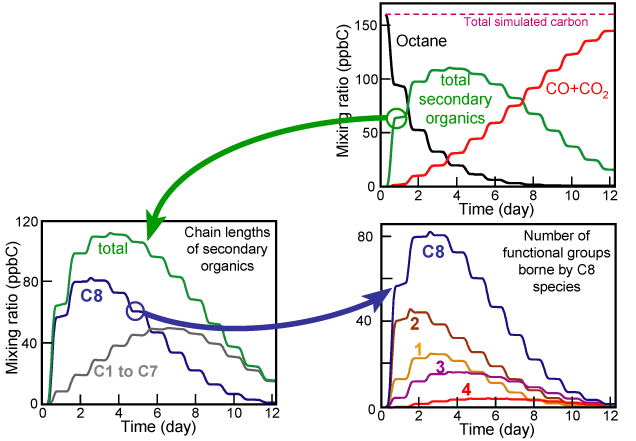 Figure 3 Figure 3
|
|





