- Basic formula
- Formula with functionnals groups
- Aromatics
- Examples
- Basic formula
The way to write the chemical formula is almost the same as the SMILES except that you
have to write all the hydrogen atoms. Atoms are represented by the standard abbreviation
of the chemical elements and functionnal
groups must be between brackets.
The method is to write each carbon of the main string with his hydrogen. For example,
propane should be written : CH3CH2CH3.
Methyl groups must be between brackets like this : CH3CH(CH3)CH3 (methyl 2 propane).
A double-bonded carbon is written 'Cd' and the double-bond is represented as '='. Example of the isoprene: CdH2=Cd(CH3)CdH=CdH2.
- Formula with functionnals groups
Writing a formula with functionnals groups is not different of the basics formula. You juste have to add the function in the main chain (do
not forget to write each atom). The aldehydes should be written like this CH3CHO. The ketones should be written like this
CH3CO(CH3) (acetaldehyde). The following groups have to be into brackets : (OH), (OOH), (NO2), (ONO2), (OONO2), (O.) and (OO.).
Therefore you can write acids (e.g. CH3CO(OH), CH3CO(OOH)), alcools (e.g. CH3CH2(OH)), nitrates (e.g. CH3(ONO2))
or PAN (e.g. CH3CO(OONO2)).
- Aromatics
When you want to write a cycle, you have to choose a node. Then you start to write the formula from this node until you have finished. To
indicate the connected nodes, you just have to use a numeric suffix label. If you want to write an aromatic, use 'c' instead of 'C'.
For example benzene will be c1HcHcHcHcHc1H and cyclohexane will be C1H2CH2CH2CH2CH2C1H2. The 'c1' (or 'C1') means that the
two atoms are connected.
e.g. trinitrotoluene 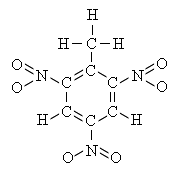
| c1(NO2)c(CH3)c(NO2)cHc(NO2)c1H |
- Examples
|
| GROUP ALLOWED in the formula |
HOW TO WRITE |
Example |
|
Skeleton (C chain)
|
| Linear C chain |
CH3, CH2, CH, C |
Pentane: CH3CH2CH2CH2CH3 |
| Branched C chain |
(CH3) |
EthylPentane: CH3CH2CH(CH2CH3)CH2CH3 |
| C=C double bond |
Cd=Cd |
Isoprene: CdH2=CdH(CH3)CdH=CdH2 |
| Oxygen in a C chain |
-O- |
Ether: CH3-O-CH3 |
Functionalities
|
| Alcohol |
(OH)
|
Ethanol: CH3CH2(OH) |
| Aldehyde |
CHO
|
Methacrolein: CdH2=Cd(CH3)CHO |
| Ketone |
CO
|
MethylGlyoxal: CH3COCHO |
| Carboxylic acid |
CO(OH)
|
Pyruvic acid: CH3COCO(OH) |
| PAN |
CO(OONO2)
|
Peroxyacetyl nitrate: CH3CO(OONO2) |
| Nitrate |
(ONO2)
|
Ethyl Nitrate: CH3CH2(ONO2) |
| Peracid |
CO(OOH)
|
CH3CH2CO(OOH) |
| Peroxy radical |
(OO.)
|
CH3CH2CH2CH(OO.)CH2CHO |
| Alkoxy radical |
(O.)
|
CH3CH2CH2CH(O.)CH2CHO |
| Hydro peroxide |
(OOH)
|
CH3CH2(OOH) |
| Peroxy acyl |
CO(OO.)
|
CH3CH2CO(OO.) |
|
|
|
|
|







